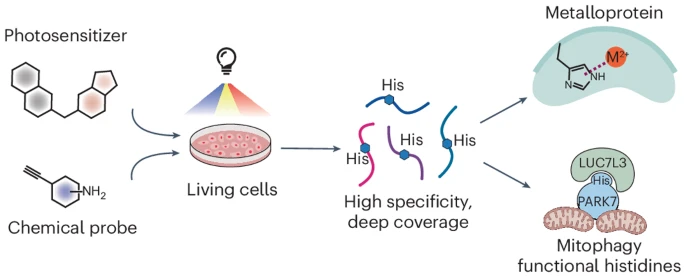
Recent advances in chemical proteomics have focused on developing chemical probes that react with nucleophilic amino acid residues. Although histidine is an attractive candidate due to its importance in enzymatic catalysis, metal binding and protein–protein interaction, its moderate nucleophilicity poses challenges. Its modification is frequently influenced by cysteine and lysine, which results in poor selectivity and narrow proteome coverage. Here we report a singlet oxygen and chemical probe relay labelling method that achieves high selectivity towards histidine. Libraries of small-molecule photosensitizers and chemical probes were screened to optimize histidine labelling, enabling histidine profiling in live cells with around 7,200 unique sites. Using NMR spectroscopy and X-ray crystallography, we characterized the reaction mechanism and the structures of the resulting products. We then applied this method to discover unannotated histidine sites key to enzymatic activity and metal binding in select metalloproteins. This method also revealed the accessibility change of histidine mediated by protein–protein interaction that influences select protein subcellular localization, underscoring its capability in discovering functional histidines.
Paper link: https://www.nature.com/articles/s41557-024-01545-6